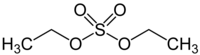
Diethyl sulfate (DES) is an organosulfur compound with the formula (C2H5)2SO4.[1][2] It occurs as a colorless, oily liquid with a faint peppermint odor. It is toxic, combustible, and likely carcinogenic chemical compound.[3][2] Diethyl sulfate is used as an ethylating agent.
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Diethyl sulfate | |
| Other names
Sulfuric acid diethyl ester
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.536 |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H10O4S | |
| Molar mass | 154.18 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.2 g/mL |
| Melting point | −25 °C (−13 °F; 248 K) |
| Boiling point | 209 °C (408 °F; 482 K) (decomposes) |
| decomposes in water | |
| Vapor pressure | 0.29 mm Hg |
| -86.8·10−6 cm3/mol | |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H302, H312, H314, H332, H340, H350 | |
| P201, P202, P260, P261, P264, P270, P271, P280, P281, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P308+P313, P310, P312, P321, P322, P330, P363, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 104 °C (219 °F; 377 K) |
| Related compounds | |
Related compounds
|
Dimethyl sulfate; diethyl sulfite |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Structure and properties
editAlthough the formula for diethyl sulfate is typically written (C2H5)2SO4, a more descriptive formula would be (C2H5O)2SO2. It is a diester of sulfuric acid. Sulfur is tetrahedral.
Diethyl sulfate hydrolyzes readily, forming ethanol and ethyl sulfate. Eventually sulfuric acid is formed with excess water.
Reactions
editDiethyl sulfate is used to an alkylating agent to prepare ethyl ethers, ethyl amines[4] and ammonium salts, and ethyl thioethers. In preparing ethyl esters of fatty acids, both equivalents of the ethyl electrophile are transferred, unlike the usual alkylation of phenoxides:[1]
- 2 RCO2Na + (C2H5O)2SO2 → 2 RCO2C2H5 + Na2SO4
Both dimethyl sulfate and diethyl sulfate react with inorganic nucleophiles as well. For example, potassium iodide gives ethyl iodide.
Preparation
editDiethyl sulfate cannot be prepared efficiently analogously to the method used for dimethyl sulfate. The reaction of oleum with diethyl ether results in excessive oxidation of the ethyl groups. Instead, diethyl sulfate is prepared in two steps starting from chlorosulfuric acid:[1]
- ClSO3H + C2H5OH → C2H5OSO3H + HCl
The resulting ethyl sulfate is then heated with sodium sulfate, leading to a redistribution reaction:
- 2 C2H5OSO3H + Na2SO4 → (C2H5O)2SO2 + 2 NaHSO4
Safety
editLike other strong alkylating agents and especially dimethyl sulfate, diethyl sulfate is toxic[2] and genotoxic.[5] It is classified as a Group 2A (probably carcinogenic to humans) carcinogen by the IARC.[6] Experimentation with animals has suggested this compound is likely carcinogenic to humans as it was implicated in the development of laryngeal cancer.[7] Evidence of the effects of this chemical compound on reproductive or developmental health is also lacking.[8]
Neutralization
editDialkyl sulfates can be rendered nontoxic by treatment with aqueous ammonia.[1]
Further reading
edit- Theodore, S.; Sai, P. S. T. (2001). "Esterification of Ethanol with Sulfuric Acid: A Kinetic Study". Canadian Journal of Chemical Engineering. 79 (1): 54–64. doi:10.1002/cjce.5450790109.
References
edit- ^ a b c d Weisenberger, Karl; Mayer, Dieter; Sandler, Stanley R. (2000). "Dialkyl Sulfates and Alkylsulfuric Acids". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a08_493. ISBN 978-3-527-30385-4.
- ^ a b c "Diethyl Sulfate | CAMEO Chemicals | NOAA". cameochemicals.noaa.gov. Retrieved 2021-03-04.
- ^ "NCI Thesaurus". ncit.nci.nih.gov. Retrieved 2021-04-02.
- ^ Buck, J. R.; Park, M.; Wang, Z.; Prudhomme, D. R.; Rizzo, C. J. (2000). "9-Ethyl-3,6-Dimethylcarbazole (DMECZ)". Organic Syntheses. 77: 153. doi:10.15227/orgsyn.077.0153.
- ^ "Agents Classified by the IARC Monographs, Volumes 1–129 – IARC Monographs on the Identification of Carcinogenic Hazards to Humans". monographs.iarc.who.int. Retrieved 2021-04-02.
- ^ IARC (1999). "Diethyl Sulfate". Summaries and Evaluations. International Agency for Research on Cancer (IARC). p. 1405.
- ^ "NCI Thesaurus". ncit.nci.nih.gov. Retrieved 2021-02-18.
- ^ "Diethyl Sulfate" (PDF). United States Environmental Protection Agency. Archived (PDF) from the original on 2016-10-14.
External links
edit- "Diethyl sulfate". Webbook. NIST.
- "DIETHYL SULFATE -- ICSC: 0570". Inchem.
- "Diethyl sulfate" (PDF). IARC Monographs. IARC. 1992.
