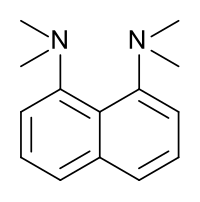
1,8-Bis(dimethylamino)naphthalene is an organic compound with the formula C10H6(NMe2)2 (Me = methyl). It is classified as a peri-naphthalene, i.e. a 1,8-disubstituted derivative of naphthalene. Owing to its unusual structure, it exhibits exceptional basicity. It is often referred by the trade name Proton Sponge, a trademark of Sigma-Aldrich.[3]
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
N1,N1,N8,N8-Tetramethylnaphthalene-1,8-diamine | |
| Other names
N,N,N′,N′-Tetramethylnaphthalene-1,8-diamine
Proton Sponge | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.039.986 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H18N2 | |
| Molar mass | 214.312 g·mol−1 |
| Appearance | White crystalline powder |
| Melting point | 47.8 °C (118.0 °F; 320.9 K) |
| Acidity (pKa) | 12.1 (in water)[1] 18.62 (in acetonitrile)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Structure and properties
editThis compound is a diamine in which the two dimethylamino groups are attached on the same side (peri position) of a naphthalene ring. This molecule has several very interesting properties; one is its very high basicity; another is its spectroscopic properties.
With a pKa of 12.34[4] for its conjugate acid in aqueous solution, 1,8-bis(dimethylamino)naphthalene is one of the strongest organic bases. However, it only absorbs protons slowly—hence the trade name. The high basicity is attributed to the relief of strain upon protonation and/or the strong interaction between the nitrogen lone pairs.[3] Additionally, although many aromatic amines such as aniline show reduced basicity (due to nitrogen being sp2 hybridized; its lone pair occupying a 2p orbital and interacting and being withdrawn by the aromatic ring), this is not possible in this molecule, as the nitrogens' methyl groups prevent its substituents from adopting a planar geometry, as this would require forcing methyl groups from each nitrogen atom into one another—thus the basicity is not reduced by this factor which is found in other molecules. It is sterically hindered, making it a weak nucleophile. Because of this combination of properties, it has been used in organic synthesis as a highly selective non-nucleophilic base.[4]
Proton sponge also exhibits a very high affinity for boron and is capable of displacing hydride from borane to form a boronium–borohydride ion pair.[5]
Preparation
editThis compound is commercially available. It may be prepared by the methylation of 1,8-diaminonaphthalene with iodomethane or dimethyl sulfate.[6]
Related compounds
editOther proton sponges
editSecond generation proton sponges are known with even higher basicity. 1,8-bis(hexamethyltriaminophosphazenyl)naphthalene or HMPN[7] is prepared from 1,8-diaminonaphthalene by reaction with tris(dimethylamino)bromophosphonium bromide in the presence of triethylamine. HMPN has a pKBH+ of 29.9 in acetonitrile which is more than 11 orders of magnitude higher than Proton Sponge.
The aromatization of an additional ring in 4,12-Dihydrogen-4,8,12-triazatriangulene is utilized by Al-Yassiri and Puchta to get a representative for a new class of Δ-shaped proton sponges.[8] This compound has a calculated proton affinity of 254 kcal/mol (B3LYP/6-311+G**) and is therefore between 1,8-Bis(dimethylamino)naphthalene and HMPN.
Hydride sponge
editThe chemical inverse of a proton sponge would be a hydride sponge. This property is exhibited by C10H6(BMe2)2, which reacts with potassium hydride to afford K[C10H6(BMe2)2H].[9]
References
edit- ^ R. W. Alder; P. S. Bowman; W. R. S. Steele & D. R. Winterman (1968). "The remarkable basicity of 1,8-bis(dimethylamino)naphthalene". Chem. Commun. (13): 723. doi:10.1039/C19680000723.
- ^ I. Kaljurand, A. Kütt, L. Sooväli, T. Rodima, V. Mäemets, I. Leito, I. A. Koppel. Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales. J. Org. Chem., 2005, 70, 1019–1028. doi:10.1021/jo048252w
- ^ a b R. W. Alder (1989). "Strain effects on amine basicities". Chem. Rev. 89 (5): 1215–1223. doi:10.1021/cr00095a015.
- ^ a b Alexander F. Pozharskii and Valery A. Ozeryanskii "Proton sponges and hydrogen transfer phenomena" Mendeleev Commun., 2012, 22, 117–124. doi:10.1016/j.mencom.2012.05.001
- ^ Légaré, Marc-André; Courtemanche, Marc-André; Fontaine, Frédéric-Georges (2014-08-28). "Lewis base activation of borane–dimethylsulfide into strongly reducing ion pairs for the transformation of carbon dioxide to methoxyboranes". Chemical Communications. 50 (77): 11362–11365. doi:10.1039/c4cc04857a. hdl:20.500.11794/29769. ISSN 1364-548X. PMID 25164269.
- ^ Vladimir I. Sorokin; Ozeryanskii, Valery A.; Pozharskii, Alexander F. (2003). "A Simple and Effective Procedure for the N-Permethylation of Amino-Substituted Naphthalenes". European Journal of Organic Chemistry. 2003 (3): 496. doi:10.1002/ejoc.200390085.
- ^ Volker Raab; Ekaterina Gauchenova; Alexei Merkoulov; Klaus Harms; Jörg Sundermeyer; Borislav Kovačević & Zvonimir B. Maksić (2005). "1,8-Bis(hexamethyltriaminophosphazenyl)naphthalene, HMPN: A Superbasic Bisphosphazene "Proton Sponge"". J. Am. Chem. Soc. 127 (45): 15738–15743. doi:10.1021/ja052647v. PMID 16277515.
- ^ Muntadar A. H. Al-Yassiri & Ralph Puchta (2023). "Predicting a New Δ-Proton Sponge-Base of 4,12-Dihydrogen-4,8,12-triazatriangulene through Proton Affinity, Aromatic Stabilization Energy, and Aromatic Magnetism". ChemPhysChem. 24 (16): e202200688. doi:10.1002/cphc.202200688. PMID 37366055.
- ^ Katz, Howard Edan (1985). "Hydride sponge: 1,8-naphtalenediylbis(dimethylborane)". Journal of the American Chemical Society. 107 (5): 1420–1421. doi:10.1021/ja00291a057.