Fluticasone/salmeterol, sold under the brand name Advair among others, is a fixed-dose combination medication containing fluticasone propionate, an inhaled corticosteroid; and salmeterol, a long-acting beta2‑adrenergic agonist.[2][3][4] It is used in the management of asthma and chronic obstructive pulmonary disease (COPD).[4] It is used by inhaling the medication into the lungs.[4]
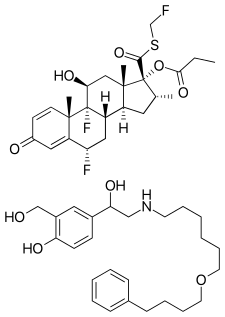 Fluticasone propionate (top) and salmeterol (bottom) | |
| Combination of | |
|---|---|
| Fluticasone propionate | Glucocorticoid |
| Salmeterol | Long-acting beta-adrenoceptor agonist |
| Clinical data | |
| Trade names | Advair, Seretide, Cyplos, others |
| AHFS/Drugs.com | FDA Professional Drug Information |
| MedlinePlus | a699063 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Inhalation |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| (verify) | |
Common side effects include thrush, headache, and cough.[5] Serious side effects may include worsening asthma, anaphylaxis, seizures, and heart problems.[5] Safety in pregnancy and breastfeeding is unclear.[6] Fluticasone, a corticosteroid, works by decreasing inflammation while salmeterol, a long-acting beta-adrenoceptor agonist (LABA), works by activating beta-2 adrenergic receptors.[5]
The combination was approved for medical use in the United States in 2000.[5] A generic version was approved in the United States in 2019.[7] In 2022, it was the 59th most commonly prescribed medication in the United States, with more than 11 million prescriptions.[8][9]
Medical uses
editFluticasone/salmeterol is indicated for the treatment of asthma.[2][3]
Fluticasone, a corticosteroid, is the anti-inflammatory component of the combination which decreases inflammation in the lungs. This leads to improvement in breathing. Salmeterol, a long-acting beta-adrenoceptor agonist, treats constriction of the airways. The combination of both is meant to be used as maintenance therapy and not as a rescue therapy for sudden symptoms.
Side effects
editThe common side effects of this combination are those of its individual drugs. For instance, the use of inhaled corticosteroids is associated with oral candidiasis, commonly known as yeast infection or thrush. Rinsing the mouth with water after inhaling the medication decreases the risk of developing this condition.
While the use of inhaled steroids and long-acting beta2‑adrenergic agonists are recommended for the resulting improvement in control of symptoms of asthma,[10] concerns have been raised that salmeterol may increase the risk of death due to asthma, and this additional risk is not reduced by the addition of inhaled steroids.[11] Other side effects from this drug combination may include increased blood pressure, change in heart rate, an irregular heartbeat, increased risk of osteoporosis, cataracts, and glaucoma.[2] Studies have demonstrated the safety of inhaled fluticasone propionate in children. A systematic review published in 2013 found no significant adverse effect on the function of the hypothalamic–pituitary–adrenal axis, growth, and bone mineral density in asthmatic children when inhaled fluticasone is used for up to three months.[12]
Mechanism of action
editFluticasone/salmeterol contains fluticasone propionate, a synthetic corticosteroid, and salmeterol, a selective long-acting beta-adrenergic receptor agonist. Fluticasone works as a potent anti-inflammatory agent, inhibiting multiple cell types such as mast cells, eosinophils, basophils, lymphocytes, macrophages, and neutrophils all of which contribute to inflammation, a large component in the pathogenesis of asthma. Salmeterol works by stimulating intracellular adenyl cyclase, which acts as a catalyst in the production of cyclic AMP. Increased cyclic AMP levels lead to a relaxation of bronchial smooth muscles. Additionally, cyclic AMP inhibits the release of mediators of immediate hypersensitivity.[2]
Society and culture
editLegal status
editIn January 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Seffalair Spiromax, intended for the treatment of asthma.[13] The applicant for this medicinal product is Teva B.V.[13] The CHMP also recommended the granting of a marketing authorization for the duplicate product BroPair Spiromax.[14] Seffalair Spiromax and BroPair Spiromax were both approved for medical use in the European Union in March 2021.[15][16][17][18]
Generic equivalents
editIn January 2019, the FDA granted Mylan the first generic approval for Advair Diskus.[7]
Civil settlements
editIn 2012, Advair was part of a larger civil settlement agreement between GlaxoSmithKline (GSK) and the United States, in which GSK agreed to pay $1.043 billion; the United States said that GSK promoted off-label uses of Advair and paid kickbacks to healthcare professionals to sell this drug, among others.[19]
References
edit- ^ "Seroflo Multihaler (Cipla Australia Pty Ltd)". Department of Health and Ages Care. Retrieved 1 April 2023.
- ^ a b c d e "Advair Diskus- fluticasone propionate and salmeterol powder". DailyMed. 30 June 2023. Retrieved 8 September 2024.
- ^ a b c "Advair HFA- fluticasone propionate and salmeterol xinafoate aerosol, metered". DailyMed. 23 May 2024. Retrieved 8 September 2024.
- ^ a b c British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 263–264. ISBN 9780857113382.
- ^ a b c d "Fluticasone and Salmeterol inhalation - FDA prescribing information, side effects and uses". Drugs.com. Retrieved 4 March 2019.
- ^ "Fluticasone / salmeterol Pregnancy and Breastfeeding Warnings". Drugs.com. Retrieved 3 March 2019.
- ^ a b "FDA approves first generic Advair Diskus". U.S. Food and Drug Administration (FDA) (Press release). Retrieved 30 January 2019.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Fluticasone; Salmeterol Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ "Guideline 101: British Guideline on the Management of Asthma". British Thoracic Society & Scottish Intercollegiate Guidelines Network (SIGN). Archived from the original on 18 April 2015.
- ^ Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE (June 2006). "Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths". Ann. Intern. Med. 144 (12): 904–12. doi:10.7326/0003-4819-144-12-200606200-00126. PMID 16754916.
- ^ Muley P, Shah M, Muley A (July 2013). "Safety of inhaled fluticasone propionate therapy for pediatric asthma - a systematic review". Current Drug Safety. 8 (3): 186–194. doi:10.2174/15748863113089990038. PMID 23859431.
- ^ a b "Seffalair Spiromax: Pending EC decision". European Medicines Agency (EMA). 1 February 2021. Archived from the original on 10 February 2021. Retrieved 1 February 2021.
- ^ "BroPair Spiromax: Pending EC decision". European Medicines Agency (EMA). 1 February 2021. Archived from the original on 13 April 2021. Retrieved 1 February 2021.
- ^ "Seffalair Spiromax EPAR". European Medicines Agency (EMA). 25 January 2021. Retrieved 23 August 2021.
- ^ "BroPair Spiromax EPAR". European Medicines Agency (EMA). 25 January 2021. Retrieved 23 August 2021.
- ^ "Seffalair Spiromax Product information". Union Register of medicinal products. Retrieved 3 March 2023.
- ^ "BroPair Spiromax Product information". Union Register of medicinal products. Retrieved 3 March 2023.
- ^ "GlaxoSmithKline to Plead Guilty and Pay $3 Billion to Resolve Fraud Allegations and Failure to Report Safety Data". Department of Justice: Office of Public Affairs. 2 July 2012.