Domperidone, sold under the brand name Motilium among others, is a dopamine antagonist medication which is used to treat nausea and vomiting and certain gastrointestinal problems like gastroparesis (delayed gastric emptying). It raises the level of prolactin in the human body and is used off label to induce and promote breast milk production.[2][10] It may be taken by mouth or rectally.[2][11][12]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Motilium, others |
| Other names | R-33812; R33812; KW-5338; KW5338; NSC-299589; NSC299589 |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration | By mouth, rectal[2] |
| Drug class | D2 receptor antagonist; Prolactin releaser |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Oral: 13–17%[2][6] Intramuscular: 90%[2] |
| Protein binding | ~92%[2] |
| Metabolism | Hepatic (CYP3A4/5) and intestinal (first-pass)[2][7] |
| Metabolites | All inactive[2][7] |
| Onset of action | 30–60 minutes[8] |
| Elimination half-life | 7–9 hours[9][2][6] |
| Excretion | Feces: 66%[2] Urine: 32%[2] Breast milk: small quantities[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.055.408 |
| Chemical and physical data | |
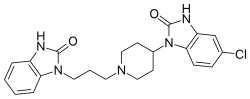
| Formula | C22H24ClN5O2 |
| Molar mass | 425.92 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 242.5 °C (468.5 °F) |
| |
| |
| (verify) | |
Side effects may include headache, anxiety, dry mouth, abdominal cramps, diarrhea, and elevated prolactin levels.[13][2][10][14] Secondary to increased prolactin levels, breast changes, milk outflow, menstrual irregularities, and hypogonadism can occur.[2][10][14] Domperidone may also cause QT prolongation and has rarely been associated with serious cardiac complications such as sudden cardiac death.[15][16][17][18] However, the risks are small and occur more with high doses.[18][19] Domperidone acts as a peripherally selective antagonist of the dopamine D2 and D3 receptors.[2][10] Due to its low entry into the brain, the side effects of domperidone are different from those of other dopamine receptor antagonists like metoclopramide and it produces little in the way of central nervous system adverse effects.[2][10] However, domperidone can nonetheless increase prolactin levels as the pituitary gland is outside of the blood–brain barrier.[20]
Domperidone was discovered in 1974 and was introduced for medical use in 1979.[21][22][23] It was developed by Janssen Pharmaceutica.[21][22] Domperidone is available over-the-counter in many countries, for instance in Europe and elsewhere throughout the world.[24][2] It is not approved for use in the United States.[25][26][2] However, it is available in the United States for people with severe and treatment-refractory gastrointestinal motility problems under an expanded access individual-patient investigational new drug application.[25] An analogue of domperidone called deudomperidone is under development for potential use in the United States and other countries.[27][28][29]
Medical uses
editNausea and vomiting
editThere is some evidence that domperidone has antiemetic activity.[2] It is recommended by the Canadian Headache Society for treatment of nausea associated with acute migraine.[30]
Gastroparesis
editGastroparesis is a medical condition characterised by delayed emptying of the stomach when there is no mechanical gastric outlet obstruction. Its cause is most commonly idiopathic, a diabetic complication or a result of abdominal surgery. The condition causes nausea, vomiting, fullness after eating, early satiety (feeling full before the meal is finished), abdominal pain and bloating. Domperidone can be used to increase the transit of food through the stomach by increasing gastrointestinal peristalsis and hence to treat gastroparesis.[2][10] It may be useful in idiopathic and diabetic gastroparesis.[31][32] However, increased rate of gastric emptying induced by drugs like domperidone does not always correlate well with relief of symptoms.[33]
Lactation
editDomperidone is used off-label in some countries to stimulate lactation or enhance breast milk production, but, as of December 2023, it is not approved for that purpose in any country, and is not approved for use in humans in the United States.[25][34] Domperidone acts as a peripheral dopamine antagonist and is hypothesized to stimulate prolactin secretion, with a 2003 study supporting that hypothesis.[26]
A 2018 meta-analysis of five randomized controlled trials found that domperidone resulted in a moderate increase of in breast milk volume for mothers of preterm infants with insufficient milk supply. The analysis also indicated that domperidone was well tolerated with no significant difference in maternal adverse events compared to placebo.[35] Domperidone has no officially established dosage for increasing milk supply, but most published studies have used 10 mg three times daily for 4 to 10 days (30 mg per day).[36]
The US Food and Drug Administration (FDA) has expressed concerns about serious adverse side effects and concerns about its effectiveness.[34] The FDA identified serious cardiac adverse events associated with domperidone use in lactating individuals, including arrhythmias, cardiac arrest, and sudden death. Additionally, discontinuation or tapering of domperidone has been linked to severe neuropsychiatric adverse events such as agitation, anxiety, and suicidal ideation. Because of these risks, the FDA strongly cautions against the use of domperidone to enhance lactation.[34]
A review by Health Canada also found a link between the sudden discontinuation or tapering of domperidone when used off-label for lactation, and psychiatric withdrawal events, particularly daily doses greater than the maximum recommended dose of 30 mg per day.[37] A 2021 study found that postpartum usage of domperidone increased across five Canadian provinces from 2004 and 2017 with usage plateauing in 2011 and a drop in usage after a 2012 Health Canada advisory warning about domperidone.[38]
Other uses
editParkinson's disease
editParkinson's disease is a degenerative neurological condition where a decrease in dopamine in the brain leads to rigidity (stiffness of movement), tremor, and other symptoms and signs. Poor gastrointestinal function, nausea, and vomiting is are major problems for people with Parkinson's disease because most medications used to treat Parkinson's disease are given by mouth. These medications, such as levodopa, can cause nausea as a side effect. Furthermore, anti-nausea drugs, such as metoclopramide, which do cross the blood–brain barrier, may worsen the extrapyramidal symptoms of Parkinson's disease. Domperidone can be used to relieve nausea and gastrointestinal symptoms in Parkinson's disease; it blocks peripheral D2 receptors but minimally crosses the blood–brain barrier in normal doses, so has no effect on the extrapyramidal symptoms of the disease.[39][40][41] In addition, domperidone may be useful in the treatment of orthostatic hypotension caused by dopaminergic therapy in people with Parkinson's disease.[42][43][44][45][46]
Other gastrointestinal uses
editDomperidone may be used in functional dyspepsia in both adults and children.[47][48] It has also been found effective in the treatment of reflux in children.[49] However some specialists consider its risks prohibitory of the treatment of infantile reflux.[50]
Available forms
editDomperidone is available for use by oral administration in the form of tablets, orally disintegrating tablets (ODTs) and suspension, and by rectal administration in the form of suppositories.[11][12] The oral tablets are available in the strength of 10 mg.[2] Domperidone has been studied for use by intramuscular injection and an intravenous formulation was previously available, but the medication is now only available in forms for oral and rectal administration.[2]
Veterinary uses
editDomperidone is used as immunotherapy to treat leishmania in dogs.[51]
Domperidone also has an FDA-approved formulation for the prevention of fescue toxicosis in periparturient mares.[52]
Contraindications
editDomperidone is contraindicated with QT-prolonging drugs like amiodarone.[53]
Side effects
editSide effects associated with domperidone include dry mouth, abdominal cramps, diarrhea, nausea, rash, itching, hives, and hyperprolactinemia (the symptoms of which may include breast enlargement, galactorrhea, breast pain/tenderness, gynecomastia, hypogonadism, and menstrual irregularities).[14]
Due to blockade of D2 receptors in the central nervous system, D2 receptor antagonists like metoclopramide and antipsychotics can also produce a variety of additional side effects including drowsiness, akathisia, restlessness, insomnia, lassitude, fatigue, extrapyramidal symptoms, dystonia, Parkinsonian symptoms, tardive dyskinesia, and depression.[2][10] However, this is not the case with domperidone, because, unlike other D2 receptor antagonists, it minimally crosses the blood–brain barrier, and for this reason, is rarely associated with such side effects.[2][10] However, domperidone theoretically might be able to produce some blockade of central D2 receptors at higher doses, in turn producing side effects similar to those of centrally permeable D2 receptor antagonists like antipsychotics.[54]
Elevated prolactin levels
editDue to D2 receptor blockade, domperidone causes hyperprolactinemia.[55] Hyperprolactinemia can suppress the secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus, in turn suppressing the secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) and resulting in hypogonadism and low levels of the sex hormones estradiol and testosterone.[56] Accordingly, 10 to 15% of females have been reported to experience mammoplasia (breast enlargement), mastodynia (breast pain/tenderness), galactorrhea (inappropriate or excessive milk production/secretion), and amenorrhea (cessation of menstrual cycles) with domperidone therapy.[55] Males may experience low libido, erectile dysfunction, and impaired spermatogenesis, as well as galactorrhea and gynecomastia.[56][57] D2 receptor antagonists like antipsychotics and domperidone may also increase the risk of prolactinomas, but more research is needed to confirm this.[58][59][60][61]
Rare reactions
editCardiac complications
editDomperidone use is associated with an increased risk of sudden cardiac death (by 70%)[15] most likely through its prolonging effect of the cardiac QT interval and ventricular arrhythmias.[62][63] The cause is thought to be blockade of hERG voltage-gated potassium channels.[16][17] The risks are dose-dependent, and appear to be greatest with high/very high doses via intravenous administration and in the elderly, as well as with drugs that interact with domperidone and increase its circulating concentrations (namely CYP3A4 inhibitors).[19][18] Conflicting reports exist, however.[64] In neonates and infants, QT prolongation is controversial and uncertain.[65][66]
UK drug regulatory authorities (MHRA) have issued the following restriction on domperidone in 2014 due to increased risk of adverse cardiac effects:[67]
Domperidone (Motilium) is associated with a small increased risk of serious cardiac side effects. Its use is now restricted to the relief of nausea and vomiting and the dosage and duration of use have been reduced. It should no longer be used for the treatment of bloating and heartburn. Domperidone is now contraindicated in those with underlying cardiac conditions and other risk factors. Patients with these conditions and patients receiving long-term treatment with domperidone should be reassessed at a routine appointment, in light of the new advice.
However, a 2015 Australian review concluded the following:[18]
Based on the results of the two TQT (the regulatory agency gold standard for assessment of QT prolongation) domperidone does not appear to be strongly associated with QT prolongation at oral doses of 20 mg QID in healthy volunteers. Further, there are limited case reports supporting an association with cardiac dysfunction, and the frequently cited case-control studies have significant flaws. While there remains an ill-defined risk at higher systemic concentrations, especially in patients with a higher baseline risk of QT prolongation, our review does not support the view that domperidone presents intolerable risk.
Possible central toxicity in infants
editIn Britain a legal case involved the death of two children of a mother whose three children had all had hypernatraemia. She was charged with poisoning the children with salt. One of the children, who was born at 28 weeks gestation with respiratory complications and had a fundoplication for gastroesophageal reflux and failure to thrive was prescribed domperidone. An advocate for the mother suggested the child may have had neuroleptic malignant syndrome as a side effect of domperidone due to the drug crossing the child's immature blood–brain barrier.[68]
Interactions
editIn healthy volunteers, the CYP3A4 inhibitor ketoconazole increased the Cmax and AUC concentrations of domperidone by 3- to 10-fold.[69] This was accompanied by a QT interval prolongation of about 10–20 milliseconds when domperidone 10 mg four times daily and ketoconazole 200 mg twice daily were administered, whereas domperidone by itself at the dosage assessed produced no such effect.[69] As such, domperidone with ketoconazole or other CYP3A4 inhibitors is a potentially dangerous combination.[69]
Pharmacology
editPharmacodynamics
editDomperidone is a peripherally selective dopamine D2 and D3 receptor antagonist.[10] It has no clinically significant interaction with the D1 receptor, unlike metoclopramide.[10] The medication provides relief from nausea by blocking D2 receptors in the chemoreceptor trigger zone and from gastrointestinal symptoms by blocking D2 receptors in the gut.[20][2] It blocks D2 receptors in the lactotrophs of the anterior pituitary gland increasing release of prolactin which in turn increases lactation.[20][70][71] Domperidone may be more useful in some patients and cause harm in others by way of the genetics of the person, such as polymorphisms in the drug transporter gene ABCB1 (which encodes P-glycoprotein), the voltage-gated potassium channel KCNH2 gene (hERG/Kv11.1), and the α1D-adrenergic receptor ADRA1D gene.[72]
Effects on prolactin levels
editA single 20 mg oral dose of domperidone has been found to increase mean serum prolactin levels (measured 90 minutes post-administration) in non-lactating women from 8.1 ng/mL to 110.9 ng/mL (a 13.7-fold increase).[10][73][74][75] This was similar to the increase in prolactin levels produced by a single 20 mg oral dose of metoclopramide (7.4 ng/mL to 124.1 ng/mL; 16.7-fold increase).[74][75] After two weeks of repeated administration (30 mg/day in both cases), the increase in prolactin levels produced by domperidone was reduced (53.2 ng/mL; 6.6-fold above baseline), but the increase in prolactin levels produced by metoclopramide, conversely, was heightened (179.6 ng/mL; 24.3-fold above baseline).[10][75] This indicates that acute and continuous administration of both domperidone and metoclopramide is effective in increasing prolactin levels, but that there are different effects on the secretion of prolactin with repeated use.[74][75] The mechanism of the difference is unknown.[75] The increase in prolactin levels observed with the two drugs was much greater in women than in men.[74][75] This appears to be due to the higher estrogen levels in women, as estrogen stimulates prolactin secretion from the pituitary gland.[76]
For comparison, normal prolactin levels in women are less than 20 ng/mL, prolactin levels peak at 100 to 300 ng/mL at parturition in pregnant women, and in lactating women, prolactin levels have been found to be 90 ng/mL at 10 days postpartum and 44 ng/mL at 180 days postpartum.[77][78]
Pharmacokinetics
editAbsorption
editThe absolute bioavailability of domperidone is low (13–17% or approximately 15%).[9][2] This is due to extensive first-pass metabolism in the intestines and liver.[9] Conversely, its bioavailability is high via intramuscular injection (90%).[2] The onset of action of domperidone taken orally is about 30 to 60 minutes.[8][2] Peak levels of domperidone following an oral dose occur after about 60 minutes.[9] Domperidone exposure increases proportionally with doses in the 10 to 20 mg dose range.[9] There is a 2- to 3-fold accumulation in levels of domperidone with frequent repeated oral administration of domperidone (four times per day (every 5 hours) for 4 days).[9] The oral bioavailability of domperidone is somewhat increased, and time to peak slightly increased when it is taken with food and bioavailability is decreased by prior concomitant administration of cimetidine and sodium bicarbonate.[9]
Distribution
editThe plasma protein binding of domperidone is 91 to 93%.[9] The tissue distribution of domperidone based on animal studies is wide, but concentrations are low in the brain.[9] The drug is a substrate for the P-glycoprotein (ABCB1) transporter, and animal studies suggest that this is the reason for the low central nervous system penetration of domperidone.[79] Small amounts of domperidone cross the placenta in animals.[9]
Metabolism
editDomperidone is extensively metabolized in the liver and intestines with oral administration.[9][7][80] This occurs via hydroxylation and N-dealkylation.[9] Domperidone is almost exclusively metabolized by CYP3A4/5, though minor contributions by CYP1A2, CYP2D6, and CYP2C8 have been reported.[80][7] CYP3A4 is the major enzyme involved in the N-dealkylation of domperidone, while CYP3A4, CYP1A2, and CYP2E1 are involved in its aromatic hydroxylation.[9] All of the metabolites of domperidone are inactive as D2 receptor ligands.[2][7] Overall and peak levels of domperidone are increased by about 2.9- and 1.5-fold in moderate hepatic impairment, respectively.[9]
Elimination
editDomperidone is eliminated 31% in urine and 66% in feces.[9] The proportion of domperidone excreted unchanged is small (10% in feces and 1% in urine).[9] The elimination half-life of domperidone is about 7 to 9 hours in healthy individuals.[9][2] However, the elimination half-life of domperidone can be prolonged to 20 hours in people with several renal dysfunction.[9][2]
Chemistry
editDomperidone is a derivative of benzimidazolinone. It is structurally related to butyrophenone neuroleptics like haloperidol.[81][82]
History
editDomperidone was synthesized at Janssen Pharmaceutica in 1974 following their research on antipsychotic drugs.[22][21] Janssen pharmacologists discovered that some antipsychotic drugs had a significant effect on dopamine receptors in the central chemoreceptor trigger zone that regulated vomiting, and started searching for a dopamine antagonist that would not pass the blood–brain barrier, thereby being free of the extrapyramidal side effects that were associated with drugs of this type.[22] This led to the discovery of domperidone as a strong antiemetic with minimal central effects.[22][83] Domperidone was patented in the United States in 1978, with the patent filed in 1976[citation needed]. In 1979, domperidone was first marketed, under the brand name Motilium, in Switzerland and West Germany.[23] Domperidone was subsequently introduced in the forms of orally disintegrating tablets (based on Zydis technology) in 1999.[84]
In April 2014, the Co-ordination Group for Mutual Recognition and Decentralised Procedures – Human (CMDh) published official press-release suggesting to restrict the use of domperidone-containing medicines. It also approved earlier published suggestions by Pharmacovigilance Risk Assessment Committee (PRAC) to use domperidone only for treating nausea and vomiting and reduce maximum daily dosage to 10mg.[11]
Society and culture
editGeneric names
editDomperidone is the generic name of the drug and its INN, USAN, BAN, and JAN.[85][86][87]
Regulatory approval
editIt was reported in 2007 that domperidone is available in 58 countries,[2] but the uses or indications of domperidone vary between nations. In Italy it is used in the treatment of gastroesophageal reflux disease and in Canada, the drug is indicated in upper gastrointestinal motility disorders and to prevent gastrointestinal symptoms associated with the use of dopamine agonist antiparkinsonian agents.[88] In the United Kingdom, domperidone is only indicated for the treatment of nausea and vomiting and the treatment duration is usually limited to 1 week.
In the United States, domperidone is not a legally marketed human drug and it is not approved for sale in the United States.[25] In June 2004, the Food and Drug Administration (FDA) issued a warning that distributing any domperidone-containing products is illegal.[25]
It is available over-the-counter to treat gastroesophageal reflux disease and functional dyspepsia in many countries, such as Ireland, the Netherlands, Italy, South Africa, Mexico, India, Chile, and China.[24]
Domperidone is not approved for use in the United States.[25] There is an exception for use in people with treatment-refractory gastrointestinal symptoms under an FDA Investigational New Drug application.[2][25]
Formulations
edit| Formulations | |||
|---|---|---|---|
| Nation | Manufacturer | Brand | Formulations |
| Australia | Janssen–Cilag | Motilium | 10 mg scored tablets[53] |
| Belgium and the Netherlands | - | Motilium | From 2013 only by prescription in Belgium.[89] |
| Bangladesh | Square | Motigut | 10 mg scored tablets |
| Bangladesh | Orion Pharma | Cosy | 10 mg scored tablets |
| Bangladesh | Astra Pharma | Domperon | 10 mg scored tablets |
| Bangladesh | - | Ridon | - |
| Canada | - | Motilium (1985–2002) | Generic brands available |
| France | Janssen | Motilium | 10 mg tablets only with prescription generic domperidone available |
| Greece | Johnson & Johnson Hellas | Cilroton | 10 mg scored tablets |
| India | Salius Pharma | Escacid DXR | pantoprazole 40 mg and domperidone SR 30 mg |
| India | FDC Pharmaceuticals | Pepcia-D | Rabeprazole 20 mg and Domperidone SR 30 mg |
| India | Rhubarb pharmaceuticals | - | domperidone 5, 10 and 20 mg tablets. |
| India | Ipca Laboratories, Mumbai | Domperi suspension | domperidone 1 mg/ml, 30 ml suspension.[90] |
| India | Torrent pharmaceuticals | Domstal | -[91] |
| India | Ozone pharmaceuticals and chemicals | Pantazone-D | 10 mg domperidone and 40 mg pantoprazole |
| India | Chimak Health Care | Pancert D | 10 mg Domperidone and 40 mg pantoprazole |
| India | Draavin Pharma | Draaci-XD | Pantaprazole 40 mg and Domperione 30 mg |
| Indonesia | Gratia Husada Farma (HUFA) | Hufadon | 10 mg caplet |
| Indonesia | Mutiara Mukti Farma | Omedom | 10 mg tablet |
| Indonesia | IFARS | Vesperum | 10 mg tablet |
| Indonesia | Dexa Medica | Vometa FT | 10 mg tablet |
| Indonesia | Sanbe | Vosedon | domperidone 5 mg/ml, 60 ml suspension |
| Iran | Abidi Pharmaceutical Co. | MOTiDON | 10 mg tablet |
| Ireland | McNeil Healthcare | Motilium | 10 mg orally disintegrating tablet (ODT) |
| Italy | - | Peridon | domperidone 10 mg tablets; 30 ml suspension |
| Lithuania | Johnson & Johnson | Motilium | - |
| Pakistan | Barrett Hodgson Pakistan | Domel | |
| Pakistan | Johnson & Johnson Pakistan | Motilium-v | domperidone 10 mg tablets; 30 ml suspension |
| Pakistan | ATCO Laboratories Limited | Vomilux | domperidone 10 mg tablets |
| Pakistan | Aspin Pharma (Pvt) Limited | Motilium | domperidone 10 mg tablets |
| Philippines | Health Saver Pharma | Abdopen | - |
| Philippines | United Laboratories, Inc. | GI Norm | - |
| Philippines | Glorious Dexa Mandaya | Vometa | domperidone 1 mg/mL oral suspension, 1 mg/mL oral drops |
| Philippines | Glorious Dexa Mandaya | Vometa FT | domperidone 10 mg fast-melting tablets |
| Portugal | Medinfar | Cinet | domperidone 1 mg/ml oral suspension (200 ml) |
| Russia | Janssen Pharmaceutica | Motilium | domperidone 10 mg film-coated tablets & ODT; 1 mg/ml suspension (100 ml) |
| - | OBL Pharm | Passagix | domperidone 10 mg film-coated tablets & chewable tablets |
| - | Dr. Reddy's Laboratories | Omez D | domperidone/omeprazole (10 mg/10 mg) |
| Saudi Arabia | JamJoom Pharmaceuticals | Dompy | Domperidone 10 mg tablets |
| Spain | Laboratorios Dr. Esteve, SA | Motilium | domperidone 1 mg/ml oral suspension (200 ml) |
| Sweden | Ebb medical | Domperidon Ebb (2013) | domperidone 10 mg ODT and peppermint |
| Syrian Arab Republic | Oubari Pharma | Motin | Domperidone 10 mg Tablets and 1 mg/ml Oral Suspension |
| Taiwan | - | Dotitone | - |
| Thailand | - | Motilium M | - |
| Turkey | Saba | Motinorm | - |
| - | GlaxoSmithKline | Motinorm | - |
Research
editDomperidone has been studied as a potential hormonal contraceptive to prevent pregnancy in women.[92]
References
edit- ^ "Motilium Product Information". Therapeutic Goods Administration (TGA). 10 June 2024. Retrieved 10 June 2024.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag Reddymasu SC, Soykan I, McCallum RW (September 2007). "Domperidone: review of pharmacology and clinical applications in gastroenterology". The American Journal of Gastroenterology. 102 (9): 2036–2045. doi:10.1111/j.1572-0241.2007.01255.x. PMID 17488253. S2CID 22575456.
- ^ "Motilium product information". Health Canada. 7 January 2002. Retrieved 10 June 2024.
- ^ "Motilium Summary of Product Characteristics (SmPC)". (emc). 11 March 2024. Retrieved 10 June 2024.
- ^ "Motilium referral". European Medicines Agency (EMA). 31 October 2000. Retrieved 10 June 2024.
- ^ a b Rose S (October 2004). Gastrointestinal and Hepatobiliary Pathophysiology. Hayes Barton Press. pp. 523–. ISBN 978-1-59377-181-2.[permanent dead link]
- ^ a b c d e Simard C, Michaud V, Gibbs B, Massé R, Lessard E, Turgeon J (2008). "Identification of the cytochrome P450 enzymes involved in the metabolism of domperidone". Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 34 (11–12): 1013–1023. doi:10.1080/00498250400015301. PMID 15801545. S2CID 27426219.
- ^ a b "Domperidone: Anti-sickness medicine used to treat nausea and vomiting". 7 January 2020.
- ^ a b c d e f g h i j k l m n o p q r "Domperidone 10mg Tablets - Summary of Product Characteristics (SMPC) - (Emc)".
- ^ a b c d e f g h i j k l Barone JA (April 1999). "Domperidone: a peripherally acting dopamine2-receptor antagonist". The Annals of Pharmacotherapy. 33 (4): 429–440. doi:10.1345/aph.18003. PMID 10332535. S2CID 39279569.
- ^ a b c "CMDh confirms recommendations on restricting use of domperidone-containing medicines" (Press release). European Medicines Agency. 25 April 2014. Archived from the original on 30 January 2016. Retrieved 31 October 2014.
- ^ a b "Motilium INSTANTS PL 13249/0028" (PDF). Medicines and Healthcare products Regulatory Agency. 23 February 2010. Archived from the original (PDF) on 13 December 2014. Retrieved 31 October 2014.
- ^ BNF 79 : March 2020. London: Royal Pharmaceutical Society. 2020. p. 444. ISBN 9780857113658.
- ^ a b c Henderson A (2003). "Domperidone. Discovering new choices for lactating mothers". AWHONN Lifelines. 7 (1): 54–60. doi:10.1177/1091592303251726. PMID 12674062.
- ^ a b Leelakanok N, Holcombe A, Schweizer ML (February 2016). "Domperidone and Risk of Ventricular Arrhythmia and Cardiac Death: A Systematic Review and Meta-analysis". Clinical Drug Investigation. 36 (2): 97–107. doi:10.1007/s40261-015-0360-0. PMID 26649742. S2CID 25601738.
- ^ a b Rossi M, Giorgi G (July 2010). "Domperidone and long QT syndrome". Current Drug Safety. 5 (3): 257–262. doi:10.2174/157488610791698334. PMID 20394569.
- ^ a b Doggrell SA, Hancox JC (January 2014). "Cardiac safety concerns for domperidone, an antiemetic and prokinetic, and galactogogue medicine" (PDF). Expert Opinion on Drug Safety. 13 (1): 131–138. doi:10.1517/14740338.2014.851193. PMID 24147629. S2CID 30668496.
- ^ a b c d Buffery PJ, Strother RM (June 2015). "Domperidone safety: a mini-review of the science of QT prolongation and clinical implications of recent global regulatory recommendations". The New Zealand Medical Journal. 128 (1416): 66–74. PMID 26117678.
- ^ a b Marzi M, Weitz D, Avila A, Molina G, Caraballo L, Piskulic L (January 2015). "[Cardiac adverse effects of domperidone in adult patients: a systematic review]". Revista Médica de Chile. 143 (1): 14–21. doi:10.4067/S0034-98872015000100002. hdl:2133/10526. PMID 25860264.
- ^ a b c Paul C, Zénut M, Dorut A, Coudoré MA, Vein J, Cardot JM, et al. (February 2015). "Use of domperidone as a galactagogue drug: a systematic review of the benefit-risk ratio". Journal of Human Lactation. 31 (1): 57–63. doi:10.1177/0890334414561265. PMID 25475074. S2CID 7978585.
- ^ a b c Wan EW, Davey K, Page-Sharp M, Hartmann PE, Simmer K, Ilett KF (August 2008). "Dose-effect study of domperidone as a galactagogue in preterm mothers with insufficient milk supply, and its transfer into milk". British Journal of Clinical Pharmacology. 66 (2): 283–289. doi:10.1111/j.1365-2125.2008.03207.x. PMC 2492930. PMID 18507654.
- ^ a b c d e Sneader W (2005). "Plant Product Analogues and Compounds Derived from Them". Drug discovery : a history. Chichester: John Wiley & Sons Ltd. p. 125. ISBN 978-0-471-89979-2.
- ^ a b "Domperidone". Pharmaceutical Manufacturing Encyclopedia, 3rd Edition (Vol. 1-4). William Andrew Publishing. 2013. p. 138. ISBN 9780815518563. Retrieved 12 December 2014.
- ^ a b Fais P, Vermiglio E, Laposata C, Lockwood R, Gottardo R, De Leo D (September 2015). "A case of sudden cardiac death following Domperidone self-medication". Forensic Science International. 254: e1–e3. doi:10.1016/j.forsciint.2015.06.004. PMID 26119456.
- ^ a b c d e f g "How to Request Domperidone for Expanded Access Use". U.S. Food and Drug Administration (FDA). 12 December 2023.
- ^ a b da Silva OP, Knoppert DC (September 2004). "Domperidone for lactating women". CMAJ. 171 (7): 725–726. doi:10.1503/cmaj.1041054. PMC 517853. PMID 15451832.
- ^ "Deudomperidone - CinRx Pharma - AdisInsight".
- ^ Heckroth M, Luckett RT, Moser C, Parajuli D, Abell TL (April 2021). "Nausea and Vomiting in 2021: A Comprehensive Update". Journal of Clinical Gastroenterology. 55 (4): 279–299. doi:10.1097/MCG.0000000000001485. PMC 7933092. PMID 33471485.
- ^ Wo JM, McCallum RW, Gonzalez Z (2021). "Antiemetic therapy for gastroparesis". Gastroparesis. Elsevier. pp. 341–359. doi:10.1016/B978-0-12-818586-5.00025-9. ISBN 9780128185865. S2CID 225132800.
- ^ Worthington I, Pringsheim T, Gawel MJ, Gladstone J, Cooper P, Dilli E, et al. (September 2013). "Canadian Headache Society Guideline: acute drug therapy for migraine headache". The Canadian Journal of Neurological Sciences. Le Journal Canadien des Sciences Neurologiques. 40 (5 Suppl 3): S1–S80. doi:10.1017/S0317167100118943. PMID 23968886.
- ^ Stevens JE, Jones KL, Rayner CK, Horowitz M (June 2013). "Pathophysiology and pharmacotherapy of gastroparesis: current and future perspectives". Expert Opinion on Pharmacotherapy. 14 (9): 1171–1186. doi:10.1517/14656566.2013.795948. PMID 23663133. S2CID 23526883.
- ^ Silvers D, Kipnes M, Broadstone V, Patterson D, Quigley EM, McCallum R, et al. (1998). "Domperidone in the management of symptoms of diabetic gastroparesis: efficacy, tolerability, and quality-of-life outcomes in a multicenter controlled trial. DOM-USA-5 Study Group". Clinical Therapeutics. 20 (3): 438–453. doi:10.1016/S0149-2918(98)80054-4. PMID 9663360.
- ^ Janssen P, Harris MS, Jones M, Masaoka T, Farré R, Törnblom H, et al. (September 2013). "The relation between symptom improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesis". The American Journal of Gastroenterology. 108 (9): 1382–1391. doi:10.1038/ajg.2013.118. PMID 24005344. S2CID 32835351.
- ^ a b c "Information about Domperidone". U.S. Food and Drug Administration (FDA). 12 December 2023. Retrieved 8 June 2024. This article incorporates text from this source, which is in the public domain.
- ^ Grzeskowiak LE, Smithers LG, Amir LH, Grivell RM (October 2018). "Domperidone for increasing breast milk volume in mothers expressing breast milk for their preterm infants: a systematic review and meta-analysis". BJOG. 125 (11). Wiley: 1371–1378. doi:10.1111/1471-0528.15177. hdl:2440/114203. PMID 29469929.
- ^ "Domperidone". Drugs and Lactation Database. Bethesda (MD): National Institute of Child Health and Human Development. 15 May 2024 [2006]. PMID 30000430.
- ^ "Assessing the Potential Risk of Psychiatric Withdrawal Events when Used for Lactation Stimulation". Drug and Health Products Portal. 8 June 2024. Retrieved 8 June 2024.
- ^ Moriello C, Paterson JM, Reynier P, Dahl M, Aibibula W, Fisher A, et al. (2021). "Off-label postpartum use of domperidone in Canada: a multidatabase cohort study". CMAJ Open. 9 (2). CMA Joule Inc.: E500–E509. doi:10.9778/cmajo.20200084. PMC 8157989. PMID 33990364.
- ^ Ramprasad C, Douglas JY, Moshiree B (December 2018). "Parkinson's Disease and Current Treatments for Its Gastrointestinal Neurogastromotility Effects". Current Treatment Options in Gastroenterology. 16 (4): 489–510. doi:10.1007/s11938-018-0201-3. PMID 30361854. S2CID 53104650.
- ^ Lertxundi U, Peral J, Mora O, Domingo-Echaburu S, Martínez-Bengoechea MJ, García-Moncó JC (March 2008). "Antidopaminergic therapy for managing comorbidities in patients with Parkinson's disease". American Journal of Health-System Pharmacy. 65 (5): 414–419. doi:10.2146/ajhp060624. PMID 18281732.
- ^ Jost WH (April 1997). "Gastrointestinal motility problems in patients with Parkinson's disease. Effects of antiparkinsonian treatment and guidelines for management". Drugs & Aging. 10 (4): 249–258. doi:10.2165/00002512-199710040-00002. PMID 9108986. S2CID 38114001.
- ^ Bacchi S, Chim I, Kramer P, Postuma RB (2017). "Domperidone for Hypotension in Parkinson's Disease: A Systematic Review". J Parkinsons Dis. 7 (4): 603–617. doi:10.3233/JPD-171209. PMID 29103053.
- ^ Papakonstantinou T, Nikolakopoulou A, Higgins JP, Egger M, Salanti G (March 2020). "CINeMA: Software for semiautomated assessment of the confidence in the results of network meta-analysis". Campbell Systematic Reviews. 16 (1): e1080. doi:10.1002/14651858.CD014883. PMC 10056828. PMID 37131978.
- ^ Lang AE (May 2001). "Acute orthostatic hypotension when starting dopamine agonist therapy in parkinson disease: the role of domperidone therapy". Arch Neurol. 58 (5): 835. doi:10.1001/archneur.58.5.835 (inactive 11 November 2024). PMID 11346387.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Luchsinger A, Grilli M, Velasco M (March 1998). "Metoclopramide and domperidone block the antihypertensive effect of bromocriptine in hypertensive patients". Am J Ther. 5 (2): 81–88. doi:10.1097/00045391-199803000-00005. PMID 10099042.
- ^ Schoffer KL, Henderson RD, O'Maley K, O'Sullivan JD (August 2007). "Nonpharmacological treatment, fludrocortisone, and domperidone for orthostatic hypotension in Parkinson's disease". Mov Disord. 22 (11): 1543–1549. doi:10.1002/mds.21428. PMID 17557339.
- ^ Xiao M, Qiu X, Yue D, Cai Y, Mo Q (2013). "Influence of hippophae rhamnoides on two appetite factors, gastric emptying and metabolic parameters, in children with functional dyspepsia" (PDF). Hellenic Journal of Nuclear Medicine. 16 (1): 38–43. PMID 23529392.
- ^ Huang X, Lv B, Zhang S, Fan YH, Meng LN (December 2012). "Itopride therapy for functional dyspepsia: a meta-analysis". World Journal of Gastroenterology. 18 (48): 7371–7377. doi:10.3748/wjg.v18.i48.7371. PMC 3544044. PMID 23326147.
- ^ Kapoor AK, Raju SM (2013). "7.2 Gastrointestinal Drugs". Illustrated Medical Pharmacology. JP Medical Ltd. p. 677. ISBN 978-9350906552. Retrieved 31 October 2014. (Google Books)
- ^ Smith R (1 August 2014). "Fear that reflux treatment for babies will be denied under new Nice guidance". The Daily Telegraph. Archived from the original on 5 October 2014. Retrieved 31 October 2014.
- ^ Travi BL, Miró G (October 2018). "Use of domperidone in canine visceral leishmaniasis: gaps in veterinary knowledge and epidemiological implications". Memórias do Instituto Oswaldo Cruz. 113 (11): e180301. doi:10.1590/0074-02760180301. PMC 6193371. PMID 30365645.
- ^ "EQUIDONE® Gel (domperidone)". dailymed.nlm.nih.gov. Retrieved 25 October 2024.
- ^ a b Swannick G. (ed.) "MIMS Australia." December 2013
- ^ Ferrier J (March 2014). "Domperidone as an unintended antipsychotic". Canadian Pharmacists Journal. 147 (2): 76–77. doi:10.1177/1715163514521969. PMC 3962062. PMID 24660005.
- ^ a b Proctor EJ, Kahn CR (2005). Joslin's Diabetes Mellitus: Edited by C. Ronald Kahn ... [et Al.]. Lippincott Williams & Wilkins. pp. 1084–. ISBN 978-0-7817-2796-9.
- ^ a b Sabanegh Jr ES (20 October 2010). Male Infertility: Problems and Solutions. Springer Science & Business Media. pp. 83–. ISBN 978-1-60761-193-6.
- ^ Briggs GG, Freeman RK, Yaffe SJ (28 March 2012). Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins. pp. 442–. ISBN 978-1-4511-5359-0.
- ^ Holt RI, Peveler RC (February 2011). "Antipsychotics and hyperprolactinaemia: mechanisms, consequences and management". Clinical Endocrinology. 74 (2): 141–147. doi:10.1111/j.1365-2265.2010.03814.x. PMID 20455888. S2CID 21617834.
- ^ Bushe CJ, Bradley A, Pendlebury J (July 2010). "A review of hyperprolactinaemia and severe mental illness: are there implications for clinical biochemistry?". Annals of Clinical Biochemistry. 47 (Pt 4): 292–300. doi:10.1258/acb.2010.010025. PMID 20592331. S2CID 21948581.
- ^ Lertxundi U, Erezuma I, Hernandez R, Medrano J, Garcia M, Aguirre C (March 2019). "Antipsychotics and pituitary tumors: an analysis of the European pharmacovigilance database (EudraVigilance)". International Clinical Psychopharmacology. 34 (2): 89–92. doi:10.1097/YIC.0000000000000247. PMID 30531551. S2CID 54476916.
- ^ Durrani US, Vasireddy S, Arshad MZ, Paracha A, Paracha MA, Waheed F, et al. (November 2023). "The Effect of Antipsychotics on Prolactinoma Growth: A Radiological and Serological Analysis". Cureus. 15 (11): e49342. doi:10.7759/cureus.49342. PMC 10748855. PMID 38143631.
- ^ van Noord C, Dieleman JP, van Herpen G, Verhamme K, Sturkenboom MC (November 2010). "Domperidone and ventricular arrhythmia or sudden cardiac death: a population-based case-control study in the Netherlands". Drug Safety. 33 (11): 1003–1014. doi:10.2165/11536840-000000000-00000. PMID 20925438. S2CID 21177240.
- ^ Johannes CB, Varas-Lorenzo C, McQuay LJ, Midkiff KD, Fife D (September 2010). "Risk of serious ventricular arrhythmia and sudden cardiac death in a cohort of users of domperidone: a nested case-control study". Pharmacoepidemiology and Drug Safety. 19 (9): 881–888. doi:10.1002/pds.2016. PMID 20652862. S2CID 20323199.
- ^ Ortiz A, Cooper CJ, Alvarez A, Gomez Y, Sarosiek I, McCallum RW (May 2015). "Cardiovascular safety profile and clinical experience with high-dose domperidone therapy for nausea and vomiting". The American Journal of the Medical Sciences. 349 (5): 421–424. doi:10.1097/MAJ.0000000000000439. PMC 4418779. PMID 25828198.
- ^ Djeddi D, Kongolo G, Lefaix C, Mounard J, Léké A (November 2008). "Effect of domperidone on QT interval in neonates". The Journal of Pediatrics. 153 (5): 663–666. doi:10.1016/j.jpeds.2008.05.013. PMID 18589449.
- ^ Günlemez A, Babaoğlu A, Arisoy AE, Türker G, Gökalp AS (January 2010). "Effect of domperidone on the QTc interval in premature infants". Journal of Perinatology. 30 (1): 50–53. doi:10.1038/jp.2009.96. PMC 2834362. PMID 19626027.
- ^ "Domperidone: Risks of cardiac side effects".
- ^ Coulthard MG, Haycock GB (January 2003). "Distinguishing between salt poisoning and hypernatraemic dehydration in children". BMJ. 326 (7381): 157–160. doi:10.1136/bmj.326.7381.157. PMC 1128889. PMID 12531853.
- ^ a b c Aronson JK (27 November 2009). Meyler's Side Effects of Antimicrobial Drugs. Elsevier. pp. 2244–. ISBN 978-0-08-093293-4.
- ^ Saeb-Parsy K. "Instant pharmacology." John Wiley & Sons, 1999 ISBN 0471976393, 9780471976394 p216.
- ^ Sakamoto Y, Kato S, Sekino Y, Sakai E, Uchiyama T, Iida H, et al. (2011). "Effects of domperidone on gastric emptying: a crossover study using a continuous real-time 13C breath test (BreathID system)". Hepato-Gastroenterology. 58 (106): 637–641. PMID 21661445.
- ^ Parkman HP, Jacobs MR, Mishra A, Hurdle JA, Sachdeva P, Gaughan JP, et al. (January 2011). "Domperidone treatment for gastroparesis: demographic and pharmacogenetic characterization of clinical efficacy and side-effects". Digestive Diseases and Sciences. 56 (1): 115–124. doi:10.1007/s10620-010-1472-2. PMID 21063774. S2CID 39632855.
- ^ Gabay MP (August 2002). "Galactogogues: medications that induce lactation". Journal of Human Lactation. 18 (3): 274–279. doi:10.1177/089033440201800311. PMID 12192964. S2CID 29261467.
- ^ a b c d Hofmeyr GJ, Van Iddekinge B, Blott JA (February 1985). "Domperidone: secretion in breast milk and effect on puerperal prolactin levels". British Journal of Obstetrics and Gynaecology. 92 (2): 141–144. doi:10.1111/j.1471-0528.1985.tb01065.x. PMID 3882143. S2CID 25489895.
- ^ a b c d e f Brouwers JR, Assies J, Wiersinga WM, Huizing G, Tytgat GN (May 1980). "Plasma prolactin levels after acute and subchronic oral administration of domperidone and of metoclopramide: a cross-over study in healthy volunteers". Clinical Endocrinology. 12 (5): 435–440. doi:10.1111/j.1365-2265.1980.tb02733.x. PMID 7428183. S2CID 27266775.
- ^ Fujino T, Kato H, Yamashita S, Aramaki S, Morioka H, Koresawa M, et al. (August 1980). "Effects of domperidone on serum prolactin levels in human beings". Endocrinologia Japonica. 27 (4): 521–525. doi:10.1507/endocrj1954.27.521. PMID 7460861.
- ^ Riordan J (January 2005). Breastfeeding and Human Lactation. Jones & Bartlett Learning. pp. 76–. ISBN 978-0-7637-4585-1.
- ^ Becker KL (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 147–. ISBN 978-0-7817-1750-2.
- ^ Bardal SK, Waechter JE, Martin DS (2011). Applied Pharmacology. Elsevier Health Sciences. pp. 184–. ISBN 978-1-4377-0310-8.
- ^ a b Youssef AS, Parkman HP, Nagar S (November 2015). "Drug-drug interactions in pharmacologic management of gastroparesis". Neurogastroenterology and Motility. 27 (11): 1528–1541. doi:10.1111/nmo.12614. PMID 26059917. S2CID 34728070.
- ^ Hospital Formulary. HFM Publishing Corporation. 1991. p. 171.
Domperidone, a benzimidazole derivative, is structurally related to the butyrophenone tranquilizers (eg, haloperidol (Haldol, Halperon]).
- ^ Biggio G, Costa E, Spano PF (22 October 2013). Receptors as Supramolecular Entities: Proceedings of the Biannual Capo Boi Conference, Cagliari, Italy, 7-10 June 1981. Elsevier Science. pp. 3–. ISBN 978-1-4831-5550-0.
- ^ Corsini GU (2010). "Apomorphine: from experimental tool to therapeutic aid" (PDF). In Ban TA, Healy D, Shorter E (eds.). The Triumph of Psychopharacology and the Story of CINP. CINP. p. 54. ISBN 978-9634081814. Archived from the original (PDF) on 1 November 2014.
- ^ Rathbone MJ, Hadgraft J, Roberts MS (2002). "The Zydis Oral Fast-Dissolving Dosage Form". Modified-Release Drug Delivery Technology. CRC Press. p. 200. ISBN 9780824708696. Retrieved 31 October 2014.
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 466–. ISBN 978-1-4757-2085-3.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 366–. ISBN 978-3-88763-075-1.
- ^ "Domperidone". Drugs.com.
- ^ "Domperidone - heart rate and rhythm disorders." Canadian adverse reactions newsletter. Government of Canada. January 2007 17(1)
- ^ "Motilium nog enkel op voorschrift". standaard.be. 7 May 2013. Retrieved 3 October 2013.
- ^ "ipcalabs.com". ipcalabs.com. Retrieved 30 June 2013.
- ^ "torrentpharma.com". torrentpharma.com. Retrieved 30 June 2013.
- ^ Hofmeyr GJ, Van Iddekinge B, Van Der Walt LA (1985). "Effect of domperidone-induced hyperprolactinaemia on the menstrual cycle; a placebo-controlled study". Journal of Obstetrics and Gynaecology. 5 (4): 263–264. doi:10.3109/01443618509067772. ISSN 0144-3615.