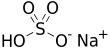Sodium bisulfate, also known as sodium hydrogen sulfate,[a] is the sodium salt of the bisulfate anion, with the molecular formula NaHSO4. Sodium bisulfate is an acid salt formed by partial neutralization of sulfuric acid by an equivalent of sodium base, typically in the form of either sodium hydroxide (lye) or sodium chloride (table salt). It is a dry granular product that can be safely shipped and stored. The anhydrous form is hygroscopic. Solutions of sodium bisulfate are acidic, with a 1M solution having a pH of slightly below 1.
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Sodium hydrogen sulfate
| |||
| Other names
Sodium acid sulfate
Bisulfate of soda (sodiooxy)sulfonic acid | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.787 | ||
| EC Number |
| ||
| E number | E514(ii) (acidity regulators, ...) | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII |
| ||
| UN number | 2837 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| NaHSO4 | |||
| Molar mass | 120.06 g/mol (anhydrous) 138.07 g/mol (monohydrate) | ||
| Appearance | white solid | ||
| Density | 2.742 g/cm3 (anhydrous) 1.8 g/cm3 (monohydrate) | ||
| Melting point | 58.5 °C (137.3 °F; 331.6 K) (monohydrate) 315 °C (anhydrous) | ||
| Boiling point | decomposes to Na2S2O7 (+ H2O) at 315 °C (599 °F; 588 K) | ||
| 28.5 g/100 mL (25 °C) 100 g/100 mL (100 °C) | |||
| Solubility | Insoluble in ammonia | ||
| Acidity (pKa) | 1.99 | ||
| Structure | |||
| triclinic (anhydrous) monoclinic (monohydrate) | |||
| Hazards | |||
| GHS labelling: | |||

| |||
| Danger | |||
| H318 | |||
| P280, P305+P351+P338, P310 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Other anions
|
Sodium sulfate | ||
Other cations
|
Potassium bisulfate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Production
editSodium bisulfate is produced as an intermediate in the Mannheim process, an industrial process involving the reaction of sodium chloride and sulfuric acid:[1]
- NaCl + H2SO4 → HCl + NaHSO4
The process for the formation of sodium bisulfate is highly exothermic. The liquid sodium bisulfate is sprayed and cooled so that it forms a solid bead. The hydrogen chloride gas is dissolved in water to produce hydrochloric acid as a useful coproduct of the reaction.
Sodium bisulfate can be generated as a byproduct of the production of many other mineral acids via the reaction of their sodium salts with an excess of sulfuric acid:
- NaX + H2SO4 → NaHSO4 + HX ( X− = CN−, NO3−, ClO4−)
The acids HX produced have a lower boiling point than the reactants and are separated from the reaction mixture by distillation.
Chemical reactions
editHydrated sodium bisulfate dehydrates at 58 °C (136 °F) at which point it separates from the water molecule attached to it. Once cooled again, it is freshly hygroscopic. Heating sodium bisulfate to 280 °C (536 °F) produces sodium pyrosulfate, another colorless salt:[1]
- 2 NaHSO4 → Na2S2O7 + H2O
Uses
editSodium bisulfate is used primarily to lower pH. It is also used in metal finishing, cleaning products,[2] and to lower the pH of water for effective chlorination in swimming pools and hot tubs.[3] Sodium bisulfate is also AAFCO approved as a general-use feed additive, including use in poultry feed[4] and companion animal food.[5] It is used as a urine acidifier to reduce urinary stones in cats.[6]
It is highly toxic to certain echinoderms, but fairly harmless to most other life forms; so it is used in controlling outbreaks of crown-of-thorns starfish.
Sodium bisulfate was the primary active ingredient in the toilet bowl cleaners Vanish and Sani-Flush, both now discontinued.[7]
In the textiles industry, it is sometimes applied to velvet cloth made with a silk backing and a pile of cellulose-based fiber (rayon, cotton, hemp, etc.) to create "burnout velvet": the sodium bisulfate, when applied to such a fabric and heated, causes the cellulose-based fibers to become brittle and flake away, leaving burned-out areas in the finished material, usually in attractive patterns.[8]
Sodium bisulfate is the active ingredient in some granular poultry litter treatments used to control ammonia.[9] Sodium bisulfate has also been shown to significantly reduce the concentration of Campylobacter and Salmonella in chicken houses.[10]
Sodium bisulfate is sometimes used as the active ingredient in flocculant tablets, a step in soil and water quality test kits.[11]
In food
editSodium bisulfate is used as a food additive to leaven cake mixes (make them rise)[12] as well as being used in meat and poultry processing and most recently in browning prevention of fresh-cut produce.[13] Sodium bisulfate is considered generally recognized as safe (GRAS) by the FDA and has been named to the EPA Safer Choice[14] Safer Chemicals Ingredients List. The food-grade product also meets the requirements set out in the Food Chemicals Codex. It is denoted by E number E514ii in the EU and is also approved for use in Australia, New Zealand, Canada, and Mexico.[15] where it is listed as additive 514. Food grade sodium bisulfate is used in a variety of food products, including beverages, dressings, sauces, and fillings. It has many synonyms including[16] bisulfate of soda, sodium acid sulfate, mono sodium hydrogen sulfate, sodium hydrogen sulfate, sodium hydrosulfate, and sulfuric acid sodium salt (1:1).
Sodium bisulfate lowers the pH without creating a sour taste, and has been used in the place of citric, malic, or phosphoric acids that are commercially available,[9] and it can also be used as an anti-browning agent.[17]
Notes
edit- ^ The prefix "bi" in "bisulfate" comes from an outdated naming system and is based on the observation that there is two times as much sulfate (SO4) in sodium bisulfate (NaHSO4) and other bisulfates as in sodium sulfate (Na2SO4) and other sulfates.
The "bi" refers to the presence of the hydrogen.
References
edit- ^ a b Helmold Plessen (2000). "Sodium Sulfates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a24_355. ISBN 978-3527306732.
- ^ John Toedt, Darrell Koza, Kathleen Van Cleef-Toedt Chemical Composition of Everyday Products p.147
- ^ "Sodium Bisulfate in Pools".
- ^ "Sodium bisulfate in Animal Feed, Agriculture". Jones-Hamilton.
- ^ "Sodium bisulfate Pet Food". Jones-Hamilton.
- ^ Spears, Julie K.; Grieshop, Christine M.; Fahey, G. C. (October 2003). "Evaluation of sodium bisulphate and phosphoric acid as urine acidifiers for cats". Archives of Animal Nutrition. 57 (5): 389–398. doi:10.1080/00039420310001607743. ISSN 1745-039X. PMID 14620912. S2CID 43171090.
- ^ SANI-FLUSH® Powder (Discontinued), Reckitt Benckiser.
- ^ Margo Singer (11 July 2007). Textile Surface Decoration: Silk and Velvet. University of Pennsylvania. p. 35. ISBN 978-0-8122-2000-1.
- ^ a b Talghari, Mariam; Behnamifar, Alireza; Rahimi, Shaban; Karimi Torshizi, Mohammad Amir; Beckstead, Robert; Grimes, Jesse L. (2020-10-01). "The effect of sodium bisulfate and coccidiostat on intestinal lesions and growth performance of Eimeria spp.–challenged broilers". Poultry Science. 99 (10): 4769–4775. doi:10.1016/j.psj.2020.06.060. ISSN 0032-5791. PMC 7598339. PMID 32988511.
- ^ "Campylobacter and Salmonella Populations Associated with Chickens Raised on Acidified Litter".
- ^ "Floc Ex Tablet MSDS" (PDF). LaMotte. 1 June 2015.
- ^ "GRAS Notice 000003: Sodium bisulfate - FDA" (PDF). FDA.
- ^ "Sodium Bisulfate - USDA" (PDF). USDA.
- ^ "EPA Safer Choice Chemical List". EPA. 11 December 2013.
- ^ "Australia New Zealand Food Standards Code - Standard 1.2.4 - Labelling of Ingredients". www.legislation.gov.au.
- ^ "Wise Eating, Made Easy". Noshly.
- ^ Ali, Hussein M.; El-Gizawy, Ahmed M.; El-Bassiouny, Rawia E. I.; Saleh, Mahmoud A. (2017-05-04). "Browning inhibition mechanisms by cysteine, ascorbic acid and citric acid, and identifying PPO-catechol-cysteine reaction products". Journal of Food Science and Technology. 52 (6): 3651–3659. doi:10.1007/s13197-014-1437-0. ISSN 0022-1155. PMC 4444905. PMID 26028748.