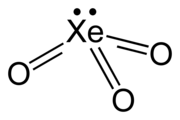
Xenon trioxide is an unstable compound of xenon in its +6 oxidation state. It is a very powerful oxidizing agent, and liberates oxygen from water slowly, accelerated by exposure to sunlight. It is dangerously explosive upon contact with organic materials. When it detonates, it releases xenon and oxygen gas.
| |

| |
| Names | |
|---|---|
| IUPAC names
Xenon trioxide
Xenon(VI) oxide | |
| Other names
Xenic anhydride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| XeO3 | |
| Molar mass | 179.288 g/mol |
| Appearance | colourless crystalline solid |
| Density | 4.55 g/cm3, solid |
| Melting point | 25 °C (77 °F; 298 K) Violent decomposition |
| Soluble (with reaction) | |
| Structure | |
| trigonal pyramidal (C3v) | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
402 kJ·mol−1[1] |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds
|
Xenon tetroxide Xenic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chemistry
editSynthesis of xenon trioxide is by aqueous hydrolysis of XeF
6:[2]
- XeF
6 + 3 H
2O → XeO
3 + 6 HF
The resulting xenon trioxide crystals are a strong oxidising agent and can oxidise most substances that are at all oxidisable. However, it is slow-acting and this reduces its usefulness.[3]
Above 25 °C, xenon trioxide is very prone to violent explosion:
When it dissolves in water, an acidic solution of xenic acid is formed:
- XeO3(aq) + H2O → H2XeO4 ⇌ H+ + HXeO−
4
This solution is stable at room temperature and lacks the explosive properties of xenon trioxide. It oxidises carboxylic acids quantitatively to carbon dioxide and water.[4]
Alternatively, it dissolves in alkaline solutions to form xenates. The HXeO−
4 anion is the predominant species in xenate solutions.[5] These are not stable and begin to disproportionate into perxenates (+8 oxidation state) and xenon and oxygen gas.[6] Solid perxenates containing XeO4−
6 have been isolated by reacting XeO
3 with an aqueous solution of hydroxides. Xenon trioxide reacts with inorganic fluorides such as KF, RbF, or CsF to form stable solids of the form MXeO
3F.[7]
Physical properties
editHydrolysis of xenon hexafluoride or xenon tetrafluoride yields a solution from which colorless XeO3 crystals can be obtained by evaporation.[2] The crystals are stable for days in dry air, but readily absorb water from humid air to form a concentrated solution. The crystal structure is orthorhombic with a = 6.163 Å, b = 8.115 Å, c = 5.234 Å, and 4 molecules per unit cell. The density is 4.55 g/cm3.[8]
| ball-and-stick model of part of the crystal structure of XeO3 |
space-filling model | coordination geometry of XeO3 |
Safety
editXeO3 should be handled with great caution. Samples have detonated when undisturbed at room temperature. Dry crystals react explosively with cellulose.[8][9]
References
edit- ^ Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A23. ISBN 978-0-618-94690-7.
- ^ a b John H. Holloway; Eric G. Hope (1998). A. G. Sykes (ed.). Recent Advances in Noble-gas Chemistry. Advances in Inorganic Chemistry, Volume 46. Academic Press. p. 65. ISBN 0-12-023646-X.
- ^ Greenwood, N.; Earnshaw, A. (1997). Chemistry of the Elements. Oxford: Butterworth-Heinemann.
- ^ Jaselskis B.; Krueger R. H. (July 1966). "Titrimetric determination of some organic acids by xenon trioxide oxidation". Talanta. 13 (7): 945–949. doi:10.1016/0039-9140(66)80192-3. PMID 18959958.
- ^ Peterson, J. L.; Claassen, H. H.; Appelman, E. H. (March 1970). "Vibrational spectra and structures of xenate(VI) and perxenate(VIII) ions in aqueous solution". Inorganic Chemistry. 9 (3): 619–621. doi:10.1021/ic50085a037.
- ^ W. Henderson (2000). Main group chemistry. Great Britain: Royal Society of Chemistry. pp. 152–153. ISBN 0-85404-617-8.
- ^ Egon Wiberg; Nils Wiberg; Arnold Frederick Holleman (2001). Inorganic chemistry. Academic Press. p. 399. ISBN 0-12-352651-5.
- ^ a b Templeton, D. H.; Zalkin, A.; Forrester, J. D.; Williamson, S. M. (1963). "Crystal and Molecular Structure of Xenon Trioxide". Journal of the American Chemical Society. 85 (6): 817. doi:10.1021/ja00889a037.
- ^ Bartlett, N.; Rao, P. R. (1963). "Xenon Hydroxide: an Experimental Hazard". Science. 139 (3554): 506. Bibcode:1963Sci...139..506B. doi:10.1126/science.139.3554.506. PMID 17843880.
